![]()
At the end of this session you should be able to:
1.Define certain concepts in research proposal development
2.Describe the steps of how to write a research proposal
When you write a research
proposal in the health sciences, you are doing so usually for a formal health
related research effort assigned to you. The assignment may be either as part
of your academic work such as an undergraduate or post-graduate dissertation,
thesis or doctoral dissertation. Research proposals are also written by individual
researchers or groups of researchers in consortia, networks, research
institutions or universities in an effort to increase research capacity and
resources by responding to call for proposals or Request For Applications
(RFAs) from funding agencies. The quality of your research proposal is therefore
critical to the future success of your final research effort. What you write in
the proposal is what you will be expected to follow step by step as you carry
out your research and prepare your finished product.
This topic will have the following sessions:
|
Session one |
Concepts and steps in proposal development |
Page number |
|
Session two |
Formats, methods and budgets in proposal development |
Page number |
SESSION 1
Concepts and steps in proposal development
1.1 Introduction
The task of writing a research proposal is enormous. It takes several months of consultations, negotiations, organizing, writing and revising before it can be considered ready. Most good proposals are written by a team of several people who will eventually carry out the work together. The team may be quite small, consisting of the investigator and one or two colleagues in the same institution, or quite big, consisting of multidisciplinary teams of researchers from different institutions or countries forming consortia for specific research agendas. Whatever the complexity of the team, the key to success in accomplishing the writing is to have one member of the team assuming the responsibility of leading the effort. Often, this is the principal investigator (PI), the individual who will have the ultimate authority and accountability for the study. The PI should be an experienced scientist with a good track record in research that may increase the likelihood of the proposal being funded. Usually, PIs delegate responsibilities for writing, setting deadlines, conducting periodic meetings of the team and assuring timely completion of tasks. This chapter assumes that readers will have been exposed to the different types of research methods and that, terms and definitions related to them will have been covered elsewhere.
1.2 SESSION OBJECTIVES
|
|
At the end of this session you should be able to: 1.Define certain concepts in research proposal development 2.Describe the steps of how to write a research proposal |
1.3 Definition of concepts
What is research?
Research:- May be defined as an activity aimed at the advancement of knowledge.
What is a Research Proposal?
A research proposal in the health sciences is a document that highlights your topic and the method you will use to formally study something related to the topic. The proposal is an effort to either confirm or test a theory or add to a scarce or non-existent body of academic literature but that asks a specific question or questions that your research effort will attempt to answer. In short, it is a document written for the advancement of knowledge. A research proposal bears specific sections and format, written to enable the researcher to obtain resources for its accomplishment. The document is then forwarded to funding agencies for funding consideration. The grant awarded to a successful proposal becomes a financial assistance which is not a loan. The phrase "research protocol” is sometimes used interchangeably with research proposal but it has a slightly different meaning. A research protocol is a detailed description of the implementation plan of a research. It is basically the methodology of a research proposal which has already been funded through solicited research.
A dissertation is an extended written treatment on a subject, usually involving research, especially by a degree candidate in partial fulfilment of a degree. Writing a successful research proposal requires scientific writing skills, negotiation skills, adherence to application instructions, budgeting and grant management skills.
What is a Research Protocol?
Research protocol:- a research protocol is a detailed description of the implementation plan of a research or study that spells out what should be done and how it should be done. Writing a protocol compels the investigator to organize, clarify and refine the study plan and enhances scientific thoroughness in its implementation. Even if funding is not required for the study, a protocol is necessary for ethical approvals and for guiding the research work.
Reasons for doing research
There are many reasons why people do research. To some researchers prestige is highest among the reasons although solving existing health problems should be the most overarching reason for doing research. Some scientists do research merely to belong to the elite club of researchers while others must obtain funds for research as part of employment contracts. In academic institutions scientists must get materials for publishing through doing research "otherwise they perish”.
|
|
In the following question choose the best alternative: A research proposal is different from a research protocol. One of the following statements is not true about the documents: a)Both the documents have the essential elements for doing the proposed research b)An investigator with research proposal writing skills is better off than one with only protocol writing skills c)Comparing a research protocol and a research proposal on the same topic the proposal will be longer than the protocol d)Comparing a research protocol and a research proposal on the same topic the proposal will be shorter than the protocol e)A research protocol cannot be submitted to funding agencies unless it is written in a proposal format |
Sources of research problems
In research proposal writing, many beginners in research are perplexed by the requirements that one must identify and define a research problem before writing a proposal. They often ask the question, "Where and how do people identify problems for research?” Although the answer to the question is encouraging, it requires critical thinking and hard work to identify and define a research problem. The following are important sources of research problems:
Routine practice: Both clinical practice and public health have day-to-day issues and problems that require solution. Every practitioner is exposed to such issues and need to be keen to identify and define them, make statements and propose research on them.
Suggestions from academic institutions: Most academic or research institutions have their own research agendas that address certain research priorities driven by scholarly thinking or national priorities and the need to do research for publishing. Academic staff and students often benefit from this source of research problems.
From national health research priority lists: Ministries of health often publish research priority areas and encourage scientists to do research in those areas since it is very likely for funds to be available if scientists can come up with good proposals. Other national bodies (research institutions) may act on behalf of the ministry to solicit proposals from the researchers.
Health priority lists of funding agencies: This is by far the leading source of research problems. Conditions for funding and motives behind the proposed research areas may differ from agency to agency with the possibilities of imposing foreign interests and policies in the research and pushing forward hidden agendas into the process.
Requests from drug/device manufacturers: Drug companies or device manufacturers have often been a good source of research problems as they commission scientists to test their products for efficacy or effectiveness.
Requests from service providers: Service providers are often unable to write good proposals and therefore they have a tendency to ask more experienced researchers to help them in solving a particular problem. In this way they become a good source of research problems.
Critical reading and review of literature: Literature review is indispensable as a source of research problems. When researchers complete their research undertakings they often make recommendations for further research in the area. This becomes a good source of a research problem.
1.4 Steps in writing proposals
Steps will include the following:
Have an idea of a research problem in mind given the situation as described above. What is the reason leading you to do the research?
Think of who is going to fund your research
Write the first draft
Pass it on to your colleagues
Make a presentation to an audience if it is part of an academic qualification
Refine your work after taking into account comments from others
Follow specific formats if any
Check methodology
Develop a budget and justify
Submit proposal
|
|
In this session we have covered; Definitions of research, research proposal and research protocol. We have listed the reasons for doing research and sources of research problems. We have also listed steps in writing proposals. |
|
|
Further reading: Read more: http://www.answers.com/topic/dissertation#ixzz2QABgpNjW |
SESSION 2
Formats, methods and budgets in proposal development
2.1 Format of a research proposal
Usually, most funding agencies provide guidelines for writing proposals. Hence, the investigator must study them well before writing the proposal. It is advisable for investigators to make contacts with the funding agencies for information that can clarify the requirements including budgetary limits and to check whether the research plan is within the agency's interests. Many of the larger donors insist on strict adherence to given formats which must be followed after the proposals have been developed, which means that the investigators must extract the relevant information from their proposals to complete such formats. Some donors distribute guidelines before the proposals are developed that the applicant follows in preparing the proposal. Yet, other donors, especially the smaller ones, offer little or no guidelines, and for them a general format is likely to suffice.
Despite the considerable variation in proposal formats, there are however, key sections, which are encountered in most research proposal formats. Even where there are no strict formats or guidelines about proposals, it is advantageous to be familiar with the key elements of a good research proposal so that essential information is not excluded when preparing one. The process helps to ensure that the proposal is presented in a logical sequence and is comprehensible to proposal reviewers who may often represent the views of the donor or funding agency.
A research proposal may be presented in as many formats as there are funding agencies. However, this session will use one general format which can easily be modified to suit the needs of most funding agencies. A good research proposal therefore has about nine distinct sections. It comprises of a title, executive summary, introduction, objectives, methodology, budget and justification, references and appendices. Large proposals should in addition have a table of contents. Each of these sections will be described in detail below:
A good research proposal must have the following general format:
1.Title
2.Executive Summary
3.Introduction
4.Statement of the problem
5.Objectives
6.Methodology
7.Budget and justification
8.References
9.Appendices
In this session we will discuss what goes into each of the above sections, one after the other and in the same order:
2.2Title
This is the topic of the study. Titles are catchy phrases that help to provide snapshot information about the study and what is to be addressed. For this reason a title must be concise and adequately descriptive. It must be short and yet clear. It must cover key variables of study in as few words as possible.
Examples of good titles:
1.The prevalence of malaria among women attending ante-natal services in Bagamoyo district. (12 words)
2.The effect of oral iron supplements on pregnancy outcome among mothers of low socio-economic status in a peri-urban area of Dar es Salaam, Tanzania: a randomized, double-blind placebo-controlled trial. (27 words)
The two titles above are examples of good titles of differing lengths. The first one is short while the second one is long. In the second one there is a deliberate attempt to make an inevitably long title short and interesting by the use of a colon and several hyphens.
2. 3 Executive Summary
This is the most critical section of any proposal as it provides guidance to busy executives as to relevance, quality and cost of the proposed study. For this reason, the summary of the proposal must have the following characteristics:
·must be short
·must be precise and yet interesting
·must state the problem and indicate why a study should be carried out
·must convince the donor of its relevance
·must indicate the study objectives
·must indicate how the research will be carried out
·must provide the total budget.
It must be emphasized that proposals may merely be rejected on the basis of a poor summary. Donors are usually busy executives and before they send out proposals to scientists for review they read the executive summaries to decide on whether or not they will be interested in such a proposal. If the summary is poorly written the proposal will be rejected at this stage. It is therefore important to pay attention to detail when writing the summary. For this reason it is the last one to be written although it comes first in the document.
3.4. Introduction
Write an introduction including a background if necessary. Describe the circumstances leading to a study in this area. The introduction provides the background of the issue to be researched on. In many proposals the literature review and problem statement are included as part of the introduction.
Literature review
In this section the literature is reviewed to know what others have written about the topic for which research is being proposed. The process helps to avoid repetition or "to re-invent the wheel”. It also helps to identify gaps in knowledge on the topic. The different methods employed by other researchers are taken into account in addressing the methodology of the proposed study.
3.5 Problem Statement
What is the research problem? How important is the problem? Its magnitude, its relevance and why it should be researched at this point in time. To answer these questions, literature review is essential. A logical sequence for presenting the problem statement would be:
·Magnitude, frequency, and distribution. Affected geographical areas and population groups affected by the problem. Ethnic and gender considerations.
·Probable causes of the problem: What is the current knowledge of the problem and its causes? Is there consensus? Is there controversy? Is there conclusive evidence?
·Possible solutions: In what ways have solutions to the problem been attempted? What has been proposed? What are the results?
·Unanswered questions: What remains to be answered? What areas have not been possible to understand, determine, verify, or test?
Make a statement of the problem and the scientific justification for the study; i.e., the basis of the need for research to generate further knowledge that will contribute to existing knowledge. The statement must be written in a way that gives empirical references to describe the situation and also clearly specifies the gaps in existing knowledge of the problem and/or the existing controversy and the nonconclusive evidence. The problem statement should make a convincing argument that there is not sufficient knowledge available to explain the problem and its possible alternative solutions, or it should make a convincing argument for the need to test what is known and taken as fact, if it is called into question by new findings or conditions.
The discussion in this section should show that the investigator has documented this problem and performed an exhaustive bibliographic review of the subject and try to answer the following question: Has the problem been addressed by other studies? If so, to what extent?
Justification of research and use of the results
This section describes the type of knowledge expected to be obtained and the intended purpose of its application. It should indicate the strategy for disseminating and using the research findings according to the potential users of the knowledge generated. The justification should answer the following:
·How does the research relate to the priorities of the region and the country?
·What knowledge and information will be obtained?
·What is the ultimate purpose that the knowledge obtained from the study will serve?
·How will the results be disseminated?
·How will the results be used, and who will be the beneficiaries?
The justification, which can be included as part of the statement of the problem or in a separate section, should make a convincing argument that the knowledge generated will be useful and generally applicable within the regional context.
Theoretical framework of the research
This is derived from the statement of the problem (presentation of empirical evidence and central question) and is the argumentation and demonstration that the "question" has a basis (grounds) for probable answer(s) and/or working hypotheses.
·Establishment of relationships (identification of the relationships between the independent variable and the response variables). What is known, and how has it been explained? Are the results conclusive? What are the bases of the question?
·How are the possible answers to the question explained and defended? What are the assumptions? What are the relationships? What are the working hypotheses?
The theoretical framework, considers the "grounds" that support the central question of the study, states the investigator's reasoning and arguments for the attempt to find the evidence that will offer an answer to the question and/or hypothesis. It also requires an exhaustive bibliographic review.
3.6Objectives
Objectives are the goals or aims of the proposed study. They are simple statements, which indicate what is to be achieved. They should be defined after the theoretical framework has been developed, and the sequence is clear between the central question and possible responses to the questions and/or working hypotheses. This is recommended because the definition of the objectives is simply the operationalization of the answers and/or hypothesis formulated by the investigator. It is recognized that not all research requires the formulation of a hypothesis for subsequent empirical verification. However, all research should explain its general and specific objectives. Objectives are the intellectual activities that the investigator will perform throughout the research process.
·General Objective: This should specify what kind of knowledge the study is expected to obtain. It should give a clear notion of what is to be described, determined, identified, compared, and, in the cases of studies with working hypotheses, confirmed.
Example
·To verify the differences in the length of time low-risk primiparous women breast-feed when they participate in the program for rooming-in at home as compared to those who do not participate.
·Specific Objectives: These disaggregate and follow logically from the general objective. They are a preliminary view of the research design meant to identify, in greater detail, the specific aims of the research, breaking down what is to be accomplished into smaller, clear, logical and measurable components. They should therefore be specific and clear, measurable, achievable, realistic and implicitly time bound (use acronym: SMART).
Examples
·To estimate the prevalence of breast-feeding in low-risk primiparous women covered by the program and the prevalence of breast-feeding in primiparous women that receive standard health care.
3.7Methodology
The methodology refers to the design and methods to be followed to achieve the set objectives. It is in this section where objectives are operationalised into activities that can be observed or measured. It explains the procedures that will be used to achieve the objectives. In this section the operational definition for the variables used should be specified in detail, along with the type of variables and the ways to measure them. In addition, the methodology should consider the study design and the techniques and procedures used to achieve the proposed objectives. A description is given below of what the investigator is expected to specify in the methodology:
Operational Definition of Variables
Based on the concepts that may be made explicit in the theoretical framework, the variables should be made operative; i.e. the investigator should clearly describe what is understood by each variable, what type of variable is being considered, and the way its values are to be reported (quantitatively, when the variable is numerical and qualitatively, when the variables do not have numerical values).
Operationalization is a process that will vary in accordance with the type of research and research design. However, the variables should be clearly defined and appropriately operationalized. Proposals will be considered incomplete if their operational aspects are vaguely formulated; for example, "The pertinent and relevant variables will be studied," "demographic and social variables will be considered," or when the statement is so imprecise that it does not allow the relevance of the variables and their use to be appraised.
Type of Study Design
The type of study and its design should be decided on the basis of its proposed objectives and the availability of resources, in addition to ethical considerations. The investigator should clearly state the type of study that will be conducted and provide a detailed explanation of its design. On this point, the investigator should also state the strategies and mechanisms that will be used to reduce or eliminate threats to the validity of the results, i.e. the so-called confounding factors (in the selection and assignment of subjects, the loss of cases, and the control of instruments and observers, etc.). These factors can be elaborated on when they are taken up in greater depth in their respective sections.
Example: An experimental controlled study will be conducted with two groups of women; those who participate in the program for rooming-in at home, and those who only receive standard care. Selection will be made of low-risk primiparous women who have been seen in the maternal and child hospital, have received at least two prenatal controls, and reside in the area of influence of the hospital. There will be two groups formed, which will be randomly assigned.
The methodology section should give details regarding the various steps to be taken to implement the study. Different study types will require different methodologies. The following is a simplified listing of the points to remember when writing the methodology section of major quantitative study types.
In clinical studies
·descriptions of study protocols
·criteria for selection of study subjects
·sample size
·description of investigations to be made
·the care to be provided
·patient follow up
·ethical issues
In Clinical trials
·protocols are much more detailed
·randomization procedures
·sample size
·blinding procedures
·number of treatment groups
·proposed agent or drug
·admission procedures and selection
·criteria for discontinuation
·patient follow up
·ethical issues
Field Studies
·describe the study area
·human population and its activities
·study population and sample size
·sociocultural background
·methods to be used (field/laboratory)
·study instruments
·ethical issues
Whatever the type of study, the following areas must be well documented
·Study design
·Study population
·Sample size
·Research instruments
·Definition of terms (i.e. what is a case?)
·Data collection and management
·Perceived constraints, biases and limitations
·Ethical issues.
Sample Selection and Size
In this section the investigator should describe the universe of study and all aspects of the selection procedures and techniques and the sample size (if this is not applicable, an explanation should be given). For both probability samples and non-probability samples (samples of convenience or grab samples) the investigator should indicate the procedure and criteria used and justify the selection and size.
In the case of studies using non-probability samples, in which subjects are selected for focus groups or as key informants, etc., the investigator should specify the selection criteria, the type of group and its size, and the procedures used to establish the group.
Here too, it is necessary to mention the selection criteria for the subjects or units of observation and the procedures to control factors relating to sample selection and size that can affect the validity of the results.
Data Collection Procedures and Instruments
The investigator should write up the procedures that will be used (population survey, in-depth interviews, non-participant observation, focus group dynamics, content analysis, etc.), how and when the procedures will be used, and the instruments that will be used to collect information (questionnaire, interview guide, observation recording form, guide for a focus group moderator, content analysis guide, etc.). Procedures or techniques that are standardized and/or documented in the literature should be described briefly, and bibliographic references should be given to sources where the details of these procedures and techniques can be found.
This section must describe in detail the procedures to be used to control the factors that undermine the validity or reliability of the results (controls for observers or persons responsible for compiling the information, and controls for the instruments).
If the use of secondary data is required, the investigator will describe their sources, content, and quality so that it will be clear that the information required for the study is available. If use is made of historical, journalistic, or other similar types of documentary sources, indication should be provided of the sources and techniques that will be used to collect and analyze the information.
Research management
What is actually going to be done first?
What are the required inputs and logistics? e.g. manpower, equipment, etc.
Your proposal should end with a brief time line, presenting estimated times for completion of the major stages of your research effort. For example:
1.Write research proposal 1 Week January 1 - 7
2.Prepare survey instrument 1 Week January 8 - 14
3.Recruit participants 2 Weeks January 8 - 22 (overlapping)
Include all the major steps and times associated with completing your research effort. Always proofread your work to make certain you are following the format required by your instructor or university, such as APA or other. Similarly, make certain your spelling of all words is correct, not simply based on your computerized spell check. Remember, there are a lot of typos you may have that pass for real words.
A timetable or schedule of events is drawn showing when each activity will be carried out (Gantt charts)
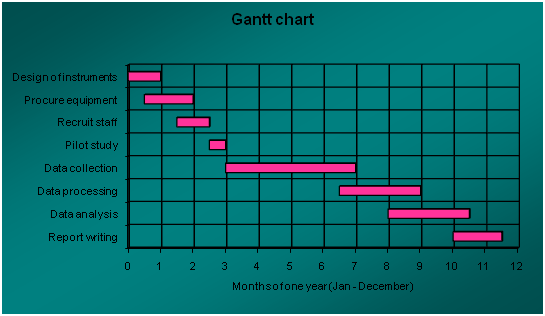 |
Monitoring
·on-going process for gathering information on implementation
·keeps track of resources
·ensures quality/quantity of activities undertaken
·identifies problems for remedial measures
·provides feed back to all concerned.
Ethical Considerations
When the research involves human subjects, this section should explicitly provide for the following aspects:
·The known benefits and risks or disadvantages for the subjects in the study.
·Exact description of the information to be delivered to the subjects of the study and when it will be communicated orally or in writing. Examples of this information include: the objectives and purposes of the study, any experimental procedures, any known short- or long-term risks, possible discomforts, expected benefits of the procedures used, duration of the studies, alternative methods for treatment if the study is a clinical trial, suspension of the study if a finding is made of negative effects or if there is sufficient evidence of positive effects that do not justify continuing with the study, and the freedom of subjects to withdraw from the study whenever they want.
·When appropriate, indicate any special incentive or treatment that subjects will receive through their participation in the study. If there is any type of remuneration, specify the amount, method of delivery, time, and reason why payment is required.
·Indicate how the information obtained from participants in the study will be kept confidential.
·List the drugs, vaccines, diagnoses, procedures, or instruments to be used, whether they are registered, unregistered, new, or currently in use in the country.
Moreover, responses are required for other ethical aspects, such as:
·In studies where personal information will be obtained from the subjects, indicate how the information will be kept confidential.
·For studies involving the participation of subjects in an experiment (experimental or quasi-experimental trials, studies of interventions, etc.), information should be provided on the free and informed consent of the participants and the strategy that will be used to obtain it.
·Brief synopsis of how the research findings will be reported and delivered to the subjects involved in the study or to other interested parties.
·Indicate and justify the inclusion, as appropriate, of children, the elderly, physical challenged, and pregnant women. Justify the non-inclusion in the study group, if appropriate, of women (of any age), an ethnic minority, racial group, etc.
·When appropriate, indicate how the appropriate balance of the two sexes will be ensured in the study groups. In addition, indicate, when appropriate, how gender inequities and discrimination and disadvantages can affect women's involvement in the research.
When studies involve human subjects, an institutional ethics committee in the country where the research will be conducted should evaluate and endorse the research. For this purpose, the form for research involving human subjects should be filled out, and care should be taken to attach the informed consent form that will be signed by the subjects involved in the study.
Plan for data Analysis
Although this item is considered under the methodology, it is suggested that the investigator treat it as a separate section. Indications are given below of what is expected from a plan of analysis. In accordance with the proposed objectives and based on the types of variables, the investigator should specify how the variables will be measured and how they will be presented (quantitative and/or qualitative), indicating the analytical models and techniques (statistical, non-statistical, or analytical techniques for non-numeric data, etc.). The investigator should provide a preliminary scheme for tabulating the data (especially for variables that are presented numerically). It is recommended that special attention be given to the key variables that will be used in the statistical models.
|
|
In completing a proposal, it is important to consider the following things:
|
3.8 Budget and justification
How much will the research cost and why? Research budget refers to the estimated amount of funds to be set aside for research activities. The budget is central to research implementation without which it is difficult for a researcher to carry out his/her research activities. A good budget provides higher chances for research funding. As such, it is critical that researchers know how to write good budgets and their justifications. Writing budgets and budget justification requires specific skills, which are lacking in most young researchers. Quite a number of proposals are returned to researchers with comments for modifications or rejection. Some of the comments are, "This budget is too ambitious!” "Unrealistic”, "Ridiculous”, "Rework the budget for reasonable estimates and resubmit for consideration”. "It is necessary that you provide elaboration and justification on the budget item of US$ 6,000 for field workers for 15 days - How many field workers and at what rate?” Sometimes you get comments like, "The budget is too small to allow the researchers to carry out the study they have proposed. They may wish to either decrease the size of their sample, number of researchers, or cut down some of the activities proposed in the study.” A budget that will generate questions among the reviewers is not realistic. Budget justification therefore entails detailing on how the funds being requested will be utilized to accomplish the project. Each budget item is explained by indicating how it will help to accomplish the project goals.
Budgets are an encouragement and incentive to researchers to work hard towards the required outputs. They are also a useful reminder of what activities are planned and have not been addressed since the budget item and amount are always indicated. In preparing budgets, one should bear in mind that funding agencies usually set limits for research project budgets and that researchers should try to respect the limits by providing budgets that are reasonable and realistic.
Budget preparation starts with a detailed examination and reference to the work plan. Location of the study, type of personnel to be used, duration of the study and material requirements are central issues in budget preparation. Requirements are specified in terms of unit cost and total cost. Use of Excel spreadsheets facilitates the calculation of total cost from unit costs and may be a great aid to allow for yearly fluctuations due to inflation and for budget items that form separate percentages of subtotals or totals such as institutional overhead costs. Use of such spreadsheets saves time especially when it comes to making small alterations in the budget that could introduce errors in calculation if a hand calculator is used. Budgets should be specified in USD (or any other convertible currency) and if not acceptable it should be specified in the currency of the funding agency.
Common budget entries may include the following examples:
1. Personnel (salaries or allowances). Professional researchers not in regular payrolls of institutions will require salaries. Some institutions hire researchers on contractual agreements and as such their salaries come from the projects they develop. At the end of the projects such researchers may extend their contracts if another project in the making matures and gets funded or their contracts are terminated and they look for employment elsewhere. Technical personnel (laboratory, data analysis, etc.) may require salaries or topping up allowances. Interviewers or research assistants will need salaries or allowances, which have to be budgeted for to meet their needs until the fieldwork is completed. Care should therefore be taken to budget adequately for personnel since they are critical to the success of data collection. Relevant support and administrative staff, drivers, etc. should not be left out in the budget because they are important contributors to the success of the project.
2. Consultants. Consultants are a special category of personnel who require substantial amounts of money to work for projects. They are expensive because they are often employed by institutions that demand a substantial proportion of the consultancy fee to compensate for gaps in output created by their absence from work in pursuit for the consultancy. They often need fees or honoraria in addition to upkeep expenses such as per diems and hotel expenses. Care should therefore be exercised before contemplating to hire consultants. Hiring of consultants is a reflection of inadequacies in the research team and may count negatively to funding possibilities. If they are acceptable by the funding agency however, the researcher should budget for them adequately.
3. Training-for-project-personnel. Interviewers or research assistants will require to be trained before work starts. The project will therefore incur expenses for their training and upkeep.
4. Facilities (purchase or rental). Very often, researchers will need working space for the project. Projects requiring laboratories will need laboratory space or will need to hire laboratory space and staff. Money for rent and utilities may be required. Research assistants may need money for renting a working space in the field. Sometimes the money budgeted for rental may be used for renovation of working space if the parent institution agrees to such arrangements.
5. Transportation. Budgeting for transportation can be tricky. Transportation can be by a variety of means including bicycles, motorcycles, cars, trucks, air or water transport. All these means may be hired, borrowed or owned. Use of one or the other means will depend on the funding agency and how much the investigator negotiates or justifies for the means. Whatever the means, it will be required by the consultants, researchers and their assistants, project managers and administrators, participants to seminars and conferences on the research project, fuel, maintenance and repair for project vehicles etc.
6. Supplies and equipment. Supplies are consumables but equipment is permanent. Some funding agencies do not want these to be combined. Supplies are stationery items like paper, pens, copier toners, diskettes, drugs, reagents and chemicals while equipment consists of computers, audiovisuals, laboratory equipment etc.
7. Dissemination of results. Success of a project is measured by its output, which should be made known to the consumers. Dissemination of research results costs money whether through holding a workshop, seminar or attending a conference. Report writing and publishing results are also a means to disseminate research results and should be budgeted for.
8. Institutional Overheads. For running costs in the institution which is housing the project. Provision should be made to protect internal institutions from exploitation and the budget should include an item related to capacity building of collaborating institutions.
9. Contingency-funds. A specified percentage (usually 5%) of the total amount should be set aside for unexpected expenses and events. Emergency treatment of project staff or dealing with accident situations may be justified if this budget item is endorsed by the funding agency. Where this expense is not acceptable, other acceptable budget items should reflect this.
10. Service component. Studies that deal with diagnosis of diseases commonly find persons requiring treatment. It will be unethical for the researcher not to provide treatment for them. For this reason, investigators should always thrive to budget and justify for this item.
|
|
To answer this question your answer should not exceed one page Write short notes to describe a typical budget of a research proposal and its justification |
3.8 References
A reference list must be included. The references relate to materials obtained during literature search and review. These will show the extent of literature review and are likely to be useful when developing manuscripts for publication.
3.9 Appendices include
-Bio-data CVs of investigators
-Institutional profiles
-Sample data sheet (questionnaires)
-Informed consent form
-Ethical clearance
-etc.
|
|
In this session we have covered; Formats, methods and budgets in proposal development. We have also covered the processes and step by step on how to write a good proposal with a high probability of getting funds. |
|
|
Further reading 1.Cummings SR, Washington AE, Ireland C, Hulley SB. Writing and funding a research proposal. In, Hulley SB and Cummings SR. eds. 1988. Designing Clinical Research; An epidemiologic approach. Williams and Wilkins, Baltimore, USA. 2.Fisher AA Foreit JR. 2002. Designing HIV/AIDS Intervention studies: An operations research handbook. Population Council. 3.Leedy PD. 1993. Practical Research: Planning and design. Macmillan Publishing Company. NewYork |
Summary