6.2 The spinal cord:
The spinal cord is the continuation of the brain and occupies the vertebral canal (Figure 6:5). It extends from the upper border of the first cervical vertebra (the atlas) to the upper border of the second lumbar vertebra. In the adult, the spinal cord occupies the upper two-thirds of the vertebral canal. It is 45cm long and ends in the conus medullaris, from the apex of which the filum terminale descends to the coccyx. Spinal puncture above L2 vertebra may result in cord damage. In the fetus, the spinal cord occupies the whole of the vertebral canal. However, the vertebral canal grows faster than the spinal cord and at birth the cord terminates at L3.
The cord is divided into segments (31) by the spinal nerves that leave it. Each segment gives off an anterior root and a posterior root. Each anterior root fuses with the corresponding posterior root at the intervertebral foramen. There are 31 pairs of spinal nerves each arising by the fusion of the anterior and posterior nerve root. The anterior root is motor (i.e. it supplies the muscles). The posterior root is sensory. The nerve roots are blocked by the analgesic solution during spinal anesthesia. The analgesic solutions block the finest fibers first. The sensory nerves, being of smaller diameter than the motor, are blocked first.
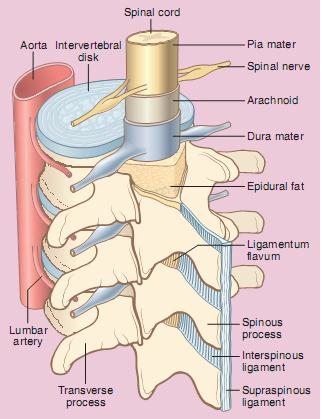
Figure 6:5 The spinal cord and surrounding structure
Coverings of the spinal cord
- The pia mater is the innermost layer, a vascular membrane which attaches intimately to the surface of the spinal cord and roots of the spinal nerves.
- The arachnoid mater is a very thin and transparent layer closely adhering to the dura mater.
- The dura mater is strong and fibrous and ending at the lower border of S2, it is separated from the bony wall of the vertebral column by the extradural space. The dural fibres are longitudinal and this must be remembered when introducing the spinal needle.
Dermatome: The area of skin supplied by the branches of each pairs of nerves (Ventral [motor] and a Dorsal [sensory]) is known as dermatome (Figure 6:6).
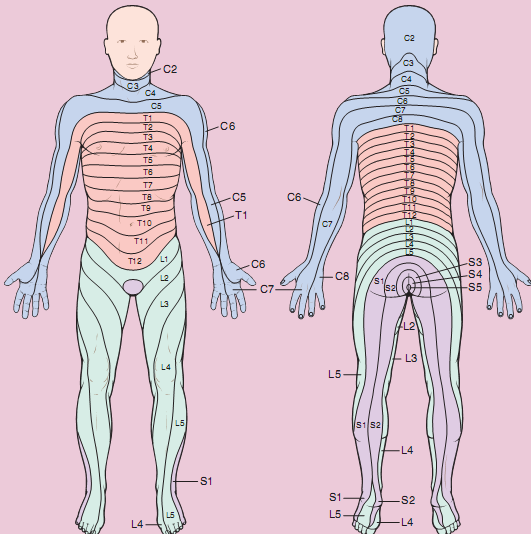
Figure 6:6 Dermatome
Segmental levels
- Perineum, S.1-4
- Inguinal region, L.1
- Umbilicus, T.10
- Sub costal arch, T.6-8
- Nipple line, T4 & 5
- Second intercostals space, T2
- Clavicle, C.3-4.
The cerebrospinal fluid: The cerebrospinal fluid occupies the space between the pia mater and the arachnoid mater. This space is called the subarachnoid space. The local anesthetic is deposited into it during spinal anesthesia. The total volume of CSF in the adult varies between 100-150 ml. CSF volume within the subarachnoid space is approximately 25-35 ml and is continually produced at a rate of 450 ml per day. It is reabsorbed into the bloodstream through the arachnoid villi and granulations. The specific gravity of CSF ranges from 1.003-1.008 at 370C, playing a crucial role in choosing the baracity of local anesthetic solution.
The weights of spinal anesthetic solutions are expressed in terms of density. Density is the weight in grams of 1 ml of a liquid. The specific gravity of a solution is a ratio: the density of the solution divided by that of water. The baricity of a spinal anesthetic solution is also a ratio: the density of the anesthetic solution divided by that of CSF.
Baricity is the most useful index of determining how spinal anesthetic solutions distribute themselves when added to CSF. If the baricity of a solution is 1.0, it is by definition isobaric; if greater than 1.0 it is hyperbaric; if less than 1.0 it is hypobaric. For spinal anesthetic solutions to be predictably hypobaric or hyperbaric in all patients requires, that their baricity be, respectively, less than 0.9990 and greater than 1.0010. The specific gravity of the local anesthetic solution can be altered by the addition of dextrose. Concentrations of 7.5% dextrose make the local anesthetic hyperbaric (heavy) relative to CSF and also reduce the rate at which it diffuses and mixes with the CSF. Isobaric and hyperbaric solutions both produce reliable blocks. Injecting hyperbaric solutions and then altering the patient's position probably produces the most controllable blocks.
Local anesthetics:
Local anesthetics are drugs which produce a reversible loss of sensation in a portion of the body by preventing nerve impulse conduction or transmission.
Local anesthetic agents can be separated chemically into basically two groups: amino ester & amino amide agents. The amino ester drugs represent the first class of local anesthetics. Cocaine, which was the first local anesthetic, introduced clinically in 1884 by Koller is an ester - type agent. Tetracaine is currently used among ester local anesthetics for spinal anesthetic. Lidocaine was the first amino amide type of local anesthetic and was introduced in to clinical practice in the mid-1940s. The available local anesthetic drugs vary somewhat in their stability, potency, duration and toxicity. Difference in these features may be related to variations in their chemical structures.
The main differences between amonoamide and amino ester types of local anesthetics are their route of metabolism & allergenic potential. Esters are hydrolyzed in plasma by the cholinesterase enzymes, where as the amide compounds undergo enzymatic degradation in the liver. The paraamino benzoic acid is one of the primary metabolites of ester-type compounds (procaine). This metabolite (degradation) product is responsible for the allergic reactions reported in a small to P-amino benzoic acid, and reports of allergic reactions to these agents are extremely rare. Amide local anesthetics are metabolized primarily by microsomal P-450 enzymes in the liver. Amides are heat-stable and can be autoclaved; esters cannot. The amides are far more stable in solution unless mixed with dextrose for spinal anesthesia.
Ideal characteristics of local anesthetics
- Low toxicity.
- Complete reversibility.
- Reasonably short latency.
- Utility for all types of local analgesia.
- Action confined to nerve tissue.
- Sufficient long duration of analgesia.
- Stability in saline & water.
- Heat stability.
- Compatibility with vasoconstrictor drugs.
Common local anesthetic agents used for spinal anesthesia
Tetracaine: is a long-acting amino ester. Currently, it is widely used in spinal anesthesia (.25-1%) when a drug of long duration is needed. It may be employed as an isobaric, hypobaric, or hyperbaric solution for spinal blockade, although hyperbaric solutions of tetracaine are probably employed most often. Tetracaine provides a relatively rapid onset of spinal anesthesia, excellent qualities of sensory anesthesia, and a profound block of motor function. Plain solutions of tetracaine produce an average duration of spinal anesthesia of 2–3 hours, whereas the addition of epinephrine can extend for 4 – 6 hours. Tetracaine also is incorporated into several topical anesthetic preparations (1-2%)
Lidocaine: was the first drug of the amino amide type to be introduced into clinical practice. Solutions of 0.5%, 1.0%, 1.5%, and 2.0% lidocaine are available for infiltration, peripheral nerve blocks, and epidural anesthesia. 5% lidocaine with 7.5% glucose is widely used for spinal anesthesia of moderate duration. Lidocaine is also used in ointment, jelly, and viscous and aerosol preparations for a variety of topical anesthetic procedures. The addition of epinephrine will significantly prolong the duration of lidocaine. Lidocaine also possesses a number of non anesthetic uses. It is widely used as an intravenous antiarrhythmic agent in patients with ventricular arrhythmias. The recommended maximum safe dose of lidocaine is 3mg/kg without epinephrine or 7mg/kg with epinephrine for peripheral nerve block.
Bupivacaine: probably had the greatest influence on the practice of regional anesthesia since the introduction of lidocaine. Bupivacaine was the first local anesthetic that combined the properties of an acceptable onset, long duration of action, profound conduction blockade, and significant separation of sensory anesthesia and motor blockade. This agent is used in concentrations of 0.125%, 0.25%, 0.5%, and 0.75 % for various regional anesthetic procedures, including infiltration, peripheral nerve blocks, and epidural and spinal anesthesia. The vascular absorption of bupivacaine is influenced to a variable extent by epinephrine, but less so than for lidocaine. Onset of spinal anesthesia with bupivacaine usually occurs within 5-15 minutes, whereas the duration of surgical anesthesia persists for 3 to 4 hours. The recommended safe dose of bupivacaine is 2mg/kg without epinephrine or 2.5mg/kg with epinephrine (if it is administered subcutaneously).
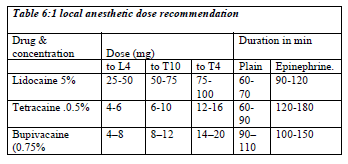
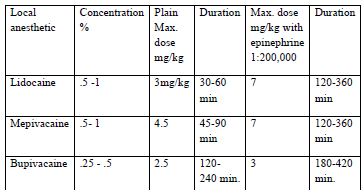
The recommended dose of local anesthetic for spinal anesthesia
The choice of spinal agents is determined by the nature and estimated time of the surgical procedure (Table 6:1). The local anesthetic should be preservative free and indicated only for spinal anesthetics. Preservative containing solution may damage the nerve in the cerebrospinal fluid. Epinephrine at the concentration of 1: 200,000 prolong the duration of action of spinal anesthesia. Adding 1:200,000 epinephrines to 2% lidocaine (bupivacaine increase 25%) will nearly double the time to resolution of blockade.
Anesthetic Potency of local anesthetic: The primary determinant of intrinsic anesthetic potency is lipid solubility which determines the agent's ability to penetrate lipid cell membrane. The nerve membrane that represents the site of action of local anesthetics consists of primarily of lipids. Additional factors include fiber size, type, and myelination, hydrogen ion balance or pH (in acidic media such as abscess the effect of local anesthetics is reduced) and vasodilator/vasoconstrictor properties (affects the rate of vascular uptake).
Nerve fiber: peripheral nerves are made up of motor, sensory and autonomic nerve fibers (axons) arranged in bundles (Table 6:2). The fibers vary considerably in size and these differences may be related to differences in function. Large diameter fibers conduct faster than smaller ones.
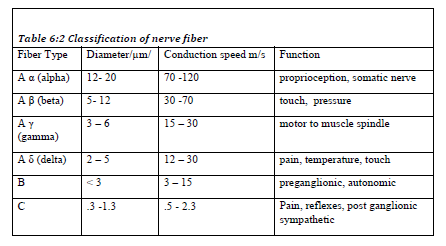
The classification of a nerve fiber impacts its sensitivity to local anesthetics. The order of susceptibility to blockade by fiber type is C fiber more susceptible then B and A fiber is more resistant. Therefore, sharp pain, and sympathetic out flow (to control blood pressure and heart rate) will be blocked first before muscle tone.
Two separate conducting pathways carrying messages related to pain are
- Comparatively fast (The acute sharp pain) – Signals are transmitted from the peripheral nerve to the spinal cord by Aδ fibers at velocity between 6 & 30 m/sec.
- Slowly conducting (slow chronic pain) Pain is transmitted by type C fibers at velocity between .5 & 2 meter per second. Because of this double system of innervations, a sudden onset of painful stimulus gives a double sensation of pain.
Factors influencing anesthetic activity clinically: The most important factors that influences the frequency of success full conduction blockade is the anesthetist's experience in the performance of the various regional anesthetic procedures. The ability to insert a needle in the appropriate anatomic location is crucial in obtaining a high degree of successful blocks. Other factors include
- Dosage of local anesthetic: An increase in the dose of local anesthetic usually results in a more profound depth of block, a prolongation of satisfactory anesthesia, and a decrease in the onset of block. An increase in the volume of local anesthetic will be beneficial in the anatomical spread of anesthesia.
- Addition of vasoconstrictor to the local anesthetic solution: Epinephrine is the most commonly used vasoconstrictor. These beneficial effects include the ability
- Prolong the duration of anesthesia by decreasing vascular absorption
- Minimize the peak level of local anesthetic in the blood and decreases the risk of toxicity
- Increase the intensity of the blockade by allowing more local anesthetic molecules to reach the nerve membrane.
Epinephrine containing local anesthetics should never be injected into end organs such as ears, nose, penis, fingers, or toes. Epinephrine may cause vasoconstriction and subsequent necrosis of tissue.
To add epinephrine to local anesthetic solutions use a 1mg/ml (1:1000) ampoule of epinephrine. Take the total volume of local anesthetic, divide it in half, and move the decimal point two places to the left. For example, 40 ml of 1% lidocaine, divide 40 by 2 and 20 is the result. Next, move the decimal point two places to the left. The result is 0.20. This is the amount of epinephrine added to the local anesthetic solution to yield a 1:200,000 concentration or dilute 1mg of adrenaline in 10cc normal saline, then take 1cc of this solution and dilute by 10 or 20cc then you will get 1:100,000 or 1:200,000
- Site of injection: Location affects the rate of diffusion, vascular absorption, and the amount of local anesthetic administered.
- Pregnancy: Hormonal changes during pregnancy are primarily responsible for the enhanced potency of local anesthetics. Mechanical factors such as dilated epidural veins, which decrease the volume of the epidural or subarachnoid space, may play a minor role in later stages of pregnancy. The spread and depth of epidural/spinal anesthesia is greater in the pregnant patient when compared to the patient who is not pregnant.
Table 6:3 Medication interaction with local anesthetics
| Ester local anesthetics |
Succinylcholine may potentiate the effects since both are dependent on pseudo-cholinesterase for metabolism. |
| Ester local anesthetics |
Cholinesterase inhibitors such as neostigmine & pyridostigmine can lead to a decrease in the metabolism of ester local anesthetics. |
| Ester local anesthetics |
Decreased pseudo-cholinesterase activity during pregnancy and postpartum period. |
| Local anesthetics in general |
Opioids and alpha adrenergic agonists potentiate the analgesic effects of local anesthetics. |
| Local anesthetics in general |
Potentiate the effects of non-depolarizing muscle relaxant blockade. |
| Lidocaine |
Cimetidine and propranolol decrease hepatic blood flow and lidocaine clearance. This acts to increase the risk of systemic toxicity. |
Table 6:4 Use of local anesthetics for other regional anesthetic techniques
| Topical-anesthesia: lidocaine, dibucaine, tetracaine, cocaine, benzocaine & EMLA (eutectic mixture of local anesthetic). |
- Topical local anesthetics provide effective, short term analgesia when applied to mucous membranes and abraded skin.
- Absorption of local anesthetics through intact skin is usually slow and unreliable and high concentrations (e.g. 20% benzocaine or 40% lignocaine) are required.
- EMLA cream is a eutectic mixture of local anesthetics which may be used to provide surface anesthesia of the skin (particularly in pediatric practice for intravenous cannulation)
- Topical anesthesia is achieved by an oropharyngeal spray of 4% lidocaine and translaryngeal injection of 3 mL of 4% lidocaine.
- Topical anesthesia with lidocaine begins to work within 30 seconds after its application and is fully active within 2 minutes, but it lasts only 20–30 minutes.
- For nasotracheal intubation, 4% cocaine or a 3-mL mixture of 4% lidocaine with 1 mL of 1% phenylephrine more commonly used today provides anesthesia while shrinking mucosa. Use of lidocaine jelly before application of other anesthetics to normal mucosa increases patient satisfaction.
|
| Infiltration techniques |
Used to provide anesthesia for minor surgical procedures
 |
| Field-block anesthesia |
Produced by subcutaneous injection of a solution of local anesthetic in order to anesthetize the region distal to the injection. For example, subcutaneous infiltration of the proximal portion of the palmar surface of the forearm results in an extensive area of cutaneous anesthesia that starts 2 to 3 cm distal to the site of injection. The same principle can be applied with particular benefit to the scalp, the anterior abdominal wall, and the lower extremity. The drugs, concentrations, and doses recommended are the same as for infiltration anesthesia. |
| Nerve Block Anesthesia |
Injection of a solution of a local anesthetic into or about individual peripheral nerves or nerve plexuses produces greater areas of anesthesia than do the techniques described above. Blockade of mixed peripheral nerves and nerve plexuses also usually anesthetizes somatic motor nerves, producing skeletal muscle relaxation, which is essential for some surgical procedures. |

